Medical Device Development: A Commitment to Quality Systems
In medical device development, the stakes are incredibly high. The level of precision and adherence to regulations at every step, as required by federal law, demands a partner with a thorough understanding of quality management systems (QMS).
kpac’s team of experts—comprising quality engineers, project managers, and biomedical engineers—guides you through the unique challenges of medical device development, from documentation and prototyping to production. With our own Quality Management System in place, we ensure that every phase meets rigorous standards. By combining deep engineering expertise with exceptional QMS design, kpac delivers unmatched efficiency and performance in the industry.
What is a QMS (Quality Management System)?
A Quality Management System (QMS) based on FDA regulations is a structured framework designed to ensure that products are consistently developed and controlled according to stringent quality standards. It covers every phase of product development, from design and manufacturing to testing and storage, with detailed procedures and documentation. This system includes key protocols for risk management, corrective and preventive actions, as well as routine audits to maintain compliance with FDA regulations.
At kpac Design, we offer customizable QMS templates that align with your product and company requirements while being fully compliant with 21 CFR 820 and ISO 13485:2016. Our QMS templates enable us to quickly implement a robust and functional system for your business. They are flexible and scalable to meet your needs and include coverage for:
- IEC 60601 Electrical Product Testing
- Templates for Class I through Class III Devices
- ISO 10993 Biocompatibility
- Device & Packaging Sterilization
- Product and Vendor Verification and Validation
Covering the most important aspects of a QMS
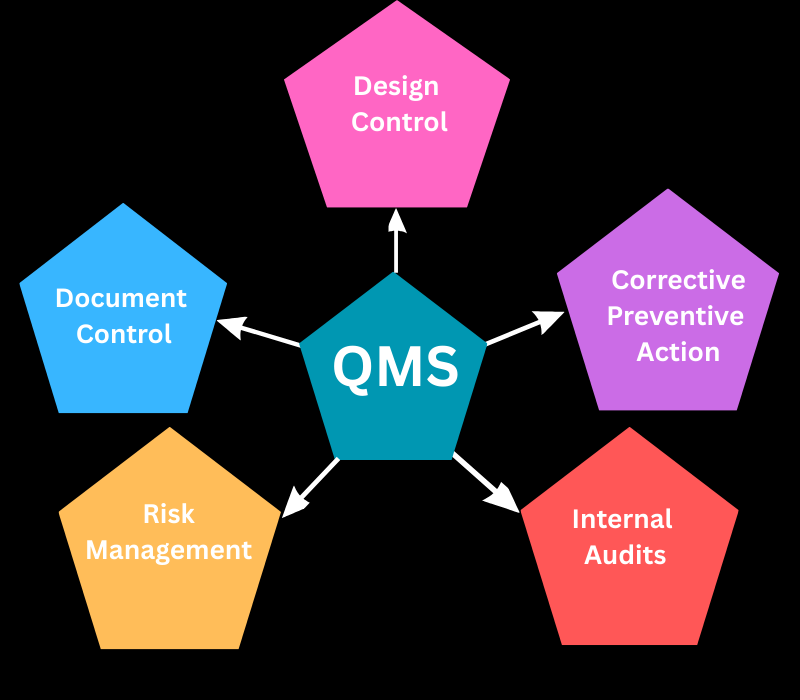
Why do you need a QMS for your medical device?
Let’s bring your project to life!
Our team of engineers is here to help. We offer free consultations and estimates.
Our Clients